Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
Abstract
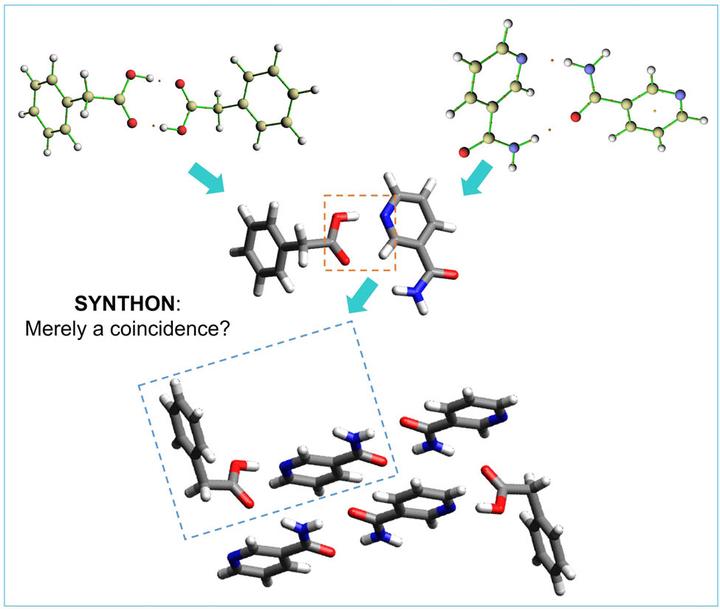
Carboxylic acid–pyridine heterosynthon (CPHS) is one of the most common synthons found in cocrystal packing. Phenylacetic acid (PYC)–nicotinamide (NIC) (PYCNIC) cocrystals were used as a computational model to assess the most important factor in the emergence of the synthon. Geometry optimization was carried out on every possible two molecules of PYC–NIC conformation based on B3LYP-D3BJ/6-311G (d,p). Various energetic parameters, including total energy, interaction energy, and hydrogen bond energy, were used to compare the existing conformation to the putative conformation. The conformation with CPHS has −53.87 kJ mol−1 of single intermolecular hydrogen bond energy (EHB), which is the strongest of all. It turns out that there is no other parameter better than EHB to describe the superiority of CPHS in PYCNIC.