Zwitterionic cocrystal of diclofenac and L-proline: Structure determination, solubility, kinetics of cocrystallization, and stability study
Abstract
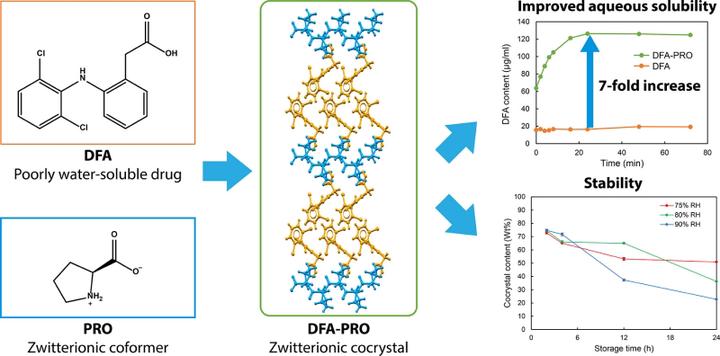
In recent decades, the design of cocrystals has developed significantly due to the unique characteristics and advantages of cocrystals, which help to improve the physicochemical properties of drugs, especially solubility. Zwitterions are attractive and interesting co-formers. However, the physicochemical properties of cocrystals with zwitterionic co-formers, i.e. zwitterionic cocrystals, have not been adequately evaluated. In this study, solid-state characterization of a newly developed zwitterionic cocrystal of diclofenac (DFA), a non-steroidal anti-inflammatory drug, and the amino acid L-proline (PRO) was performed using Fourier-transform infrared spectroscopy, differential scanning calorimetry, and powder X-ray diffraction (PXRD) analyses. In addition, the crystal structure of the cocrystal (DFA-PRO) was determined by single-crystal X-ray diffraction analysis, after which the zwitterionic structure was confirmed. The cocrystallization during co-grinding, which was investigated by PXRD, followed first-order kinetics. Furthermore, the solubility of the zwitterionic cocrystals was 7.5-times higher than that of the DFA crystals. The results indicate that the cocrystal is stable under ambient conditions; however, it hydrates and transforms into a mixture of L-proline monohydrate crystals and DFA crystals under conditions of high humidity.