Solubility improvement of epalrestat by layered structure formation via cocrystallization
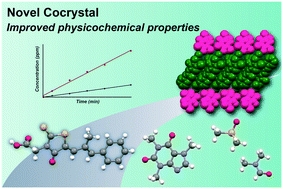
Abstract
Epalrestat, a drug for diabetic neuropathy, was able to form a cocrystal with a pharmaceutically acceptable coformer of caffeine. The cocrystal was characterized using powder X-ray diffraction and infrared spectroscopy, and the structure was determined using single crystal structure analysis. Pharmaceutically relevant physicochemical properties such as solubility, dissolution rate, and physical stability were evaluated. The cocrystal exhibited higher solubility and faster dissolution than the parent drug material. This improvement corresponded to the formation of a layered structure in the cocrystal, wherein a chain consisting of epalrestat molecules is sandwiched between caffeine molecules. The cocrystal also exhibited physical stability during a slurry experiment in most organic solvents, except in dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) solvents. In these solvents, the cocrystals underwent disproportionation into caffeine and epalrestat solvates (DMF and DMSO), and the crystal structures of epalerstat DMF and DMSO solvates are also reported in this study.