Simultaneous Improvement of Epalrestat Photostability and Solubility via Cocrystallization: A Case Study
Abstract
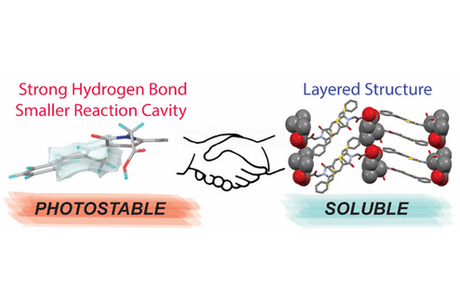
In this study, we attempt to simultaneously improve the photostability and solubility of epalrestat (a drug used for neuropathy treatment) by preparing epalrestat–betaine zwitterionic cocrystals and characterize the physicochemical property alterations accompanying their formation. Notably, we reveal that the strong hydrogen bonds between epalrestat and betaine molecules in the above cocrystals and the reduced size of the reaction cavity around epalrestat molecules prevent the E,Z to Z,Z photoisomerization of the latter, resulting in improved photostability. Furthermore, the prepared cocrystals exhibit a higher solubility and larger dissolution rate than pure epalrestat crystals due to featuring a layered structure with alternately arranged epalrestat and betaine coformer molecules.